Answer:
a. 265K
b. 0.0821
c. 7.8
Step-by-step explanation:
a. converting from celsius to kelvin: K = C + 273
converting from kelvin to celsius: C = K - 273
b. Value when working with atm is 0.0821
Value when working with torr/Hg is 62.4
c. Use the PV=nRT formula:
0.900(L) = .0821 x 0.323 x 265
0.900(L) = 7.027
L =
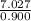
L = 7.8
good luck!!