An acyclic alkane with 7 carbon atoms is called heptane. To determine the amount of hydrogen atoms heptane has we can apply the general formula for an alkane.
The general formula for the number of hydrogen atoms in an acyclic alkane can be determined using the formula
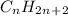 where n represents the number of carbon atoms.
where n represents the number of carbon atoms.
For heptane we substitute n = 7 into the formula:
H = 2(7) + 2
H = 14 + 2
H = 16
Answer: Therefore, an acyclic alkane with 7 carbon atoms (heptane) contains 16 hydrogen atoms.