

The relationship between the mass, the number of moles, and the molar mass is the following:
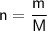
Where:
- n is the number of moles in mol.
- m is the mass in g.
- M is the molar mass in g/mol.

[ Definition: ]
The molar mass of a compound corresponds to the mass of one mole of that substance.

We have to determine the molar mass of a molecule of
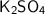 , also known as potassium sulfate.
, also known as potassium sulfate.

Using a periodic table of elements, we get:
M(K) = 39.1 g/mol
M(S) = 32 g/mol
M(O) = 16 g/mol

We are now able to calculate the molar mass of one molecule of potassium sulfate.
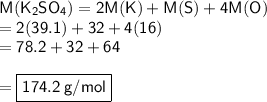

Now, let's substitute our values into the formula:
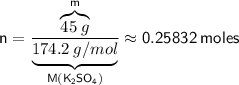

Rounding our answer to the nearest hundredth, we get:
