Step-by-step explanation:
a)
from the formula you know that
1 mol h3po4 + 3 mol naoh gives 3 moles h2o
you have
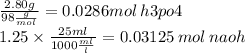
how many moles of h2o will you produce with the reactants you have?
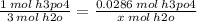
x mol h2o = 0.0858 mol
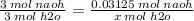
x mol h2o = 0.03125 mol
one of your reactants gives less moles of h2o , that's your limiting reactant and that means you'll get 0.03125 mol of h2o and not 0.0858 mol
so you'll have
0.03125 mol × 18 g/mol h20 = 0.5625 g h2o
b)
the mass of reactant that remains is your reactant in excess, h3po4
since you'll only produce 0.03125 mol h2o
you'll only consume x moles of h3po4
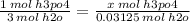
0.0104 mol = x mol h3po4
you'll have an excess of h3po4 that won't react
moles used - moles consumed = moles in excess
0.0286 mol - 0.0104 mol = 0.0182 mol
the mass of the reactant that remains is
0.0182 mol × 98g/mol = 1.78g h3po4