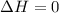For a spontaneous biochemical reaction with a zero change in entropy (
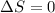 ), the relationship between enthalpy (
), the relationship between enthalpy (
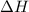 ) and temperature (
) and temperature (
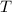 ) can be described by the equation
) can be described by the equation
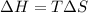 .
.
However, since
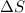 is equal to zero in this case, the equation simplifies to
is equal to zero in this case, the equation simplifies to
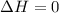 . Therefore, the correct option is:
. Therefore, the correct option is:
a.