The formula for mass/volume percent is:
 .
.
Given:
Mass of NaCl = 119 g
Total volume of the solution = 4.24 L
Plugging in the values into the formula:
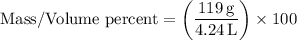 .
.
Now, let's perform the calculation:
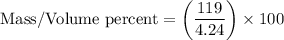 .
.
Calculating this expression, we have:
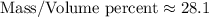 .
.
Therefore, the mass/volume percent of the NaCl solution is approximately 28.1%.
♥️