Answer: The answer is B. The pressure inside the tires increased.
Step-by-step explanation:
The relationship between the pressure, volume, and temperature of a gas is described by the ideal gas law, which is usually written as:
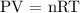
where:
-
 is the pressure,
is the pressure,
-
 is the volume,
is the volume,
-
 is the number of moles of gas,
is the number of moles of gas,
-
 is the ideal gas constant, and
is the ideal gas constant, and
-
 is the temperature (in Kelvin).
is the temperature (in Kelvin).
In this case, the volume
 and the number of moles
and the number of moles
 of air in the tires stay the same. The temperature
of air in the tires stay the same. The temperature
 is increasing. Therefore, for the equation to remain balanced, the pressure
is increasing. Therefore, for the equation to remain balanced, the pressure
 must also increase.
must also increase.
So, the answer is B. The pressure inside the tires increased.