Step-by-step explanation:
Since the element X is trivalent it has 3 valence electrons. Since the Cl ion has a charge of -1, the chloride of this element is of the form
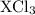 .
.
Assuming that Cl has two isotopes of mass numbers 35 and 37, it follows that
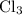 has four possible mass numbers:
has four possible mass numbers:
- 35 + 35 + 35 = 105 amu
- 35 + 35 + 37 = 107 amu
- 35 + 37 + 37 = 109 amu
- 37 + 37 + 37 = 111 amu
Notice that these four possible mass numbers all differ by exactly 2 -- the same difference observed in the peaks of the mass spectrometer.
This means that only one isotope of X is involved. If there were more than one isotope of X involved, we would expect to see more than four peaks (since there would be more than four possible combinations of
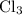 with X).
with X).
Finally, to calculate the mass number of X, we subtract the observed peak data from the known mass numbers of
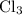 :
:
- 186 - 111 = 75 amu
- 184 - 109 = 75 amu
- 182 - 107 = 75 amu
- 180 - 105 = 75 amu
(In fact, we only really needed to calculate one of these since we already concluded that there is only one isotope of X involved!).
So, the mass number of X is 75 amu.