The final pressure will be approximately 1.19 atm when the temperature changes from 315.0 K to 150.0 K, holding the volume constant.
To solve this problem, we can use Charles' Law, which states that, at constant volume, the pressure and temperature of a gas are directly proportional.
The formula we will use is:
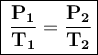
Where:
- P₁ = initial pressure = 2.50 atm
- T₁ = initial temperature = 315.0 K
- T₂ = temperature = 150.0 K
- P₂ = final pressure = ?
Solving the formula for V₂:
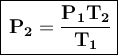
Where:
- P₁ = initial pressure
- T₁ = initial temperature
- T₂ = temperature
- P₂ = final pressure
We substitute the known values:
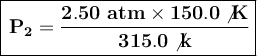

The final pressure will be approximately 1.19 atm when the temperature changes from 315.0 K to 150.0 K, holding the volume constant.