Answer:
The temperature of the gas is 293.15 K.
The temperature of the gas is 303.15 K.
The new pressure of the gas is 0.97 atm.
Step-by-step explanation:
You need to add 273.15 to the Celsius temperature to convert from Celsius to Kelvin.
So,
20.0°C + 273.15 = 293.15 K.
30.0°C + 273.15 = 303.15 K.
We can use the following equation to calculate the new pressure:
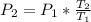
where:
- P1 is the initial pressure
- P2 is the final pressure
- T1 is the initial temperature
- T2 is the final temperature
Plugging in the values from the problem, we get:
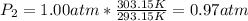
Therefore, the new pressure of the gas is 0.97 atm.