Answer:
23.90 years
Explanation:
To find the half-life of a radioactive substance, we can use the formula:
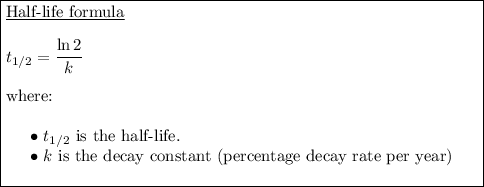
In this case, the decay constant is 2.9% or 0.029 (expressed as a decimal). Therefore, substitute k = 0.029 into the formula:
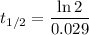
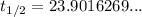
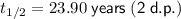
Therefore, the half-life of the radioactive substance, rounded to 2 decimal places, is approximately 23.90 years.