Answer:
Volume = 3.4 L
Step-by-step explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. =
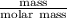
=
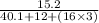
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. =
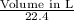
⇒
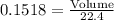
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.