1. The volume of gas is 193.548 L.
2. The temperature T of CO2 is 8.867 K.
3. The moles (n) of helium gas is 0.070 mol.
4. The temperature T of methane is 694.94 K.
5. The mass of ammonia is 0.069723 g.
6. Density of 2.3 mol of ethane gas is approximately 1.199 mol/L.
7. The temperature (T) is approximately 176.45 K
1. **Volume of Gas:** Use the ideal gas law PV = nRT to find the volume (\(V\)) when given moles (n), temperature (T), and pressure P. Convert temperature to Kelvin (K).
![\[ V = (nRT)/(P) \]](https://img.qammunity.org/2024/formulas/chemistry/college/hcxttrtsuc2aaak5gjn37iv4c2czoux2cr.png)
![\[ V = \frac{(4.3 \, \text{mol})(8.314 \, \text{J/mol}\cdot\text{K})(28 + 273.15 \, \text{K})}{165.2 \, \text{kPa}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/kjnpb3qwcelqk2xobrstrvlp3upelry3wh.png)
Therefore, the volume of gas is 193.548 L.
2. **Temperature of CO2:** Use the ideal gas law to find the temperature (\(T\)) when given pressure (\(P\)), volume (\(V\)), and mass (\(m\)).
![\[ T = (PV)/(nR) \]\\T = \frac{(968 \, \text{mmHg})(25.12 \, \text{L})}{(44.01 \, \text{g/mol})(0.0821 \, \text{L}\cdot\text{atm/mol}\cdot\text{K})} \]](https://img.qammunity.org/2024/formulas/chemistry/college/yljlmvlpcsqbk0zi9mnee78ibwnb5j2tqm.png)
Therefore the temperature T of CO2 is 8.867 K
3. **Moles of Helium Gas:** Use the ideal gas law to find moles (\(n\)) when given volume (\(V\)), temperature (\(T\)), and pressure (\(P\)). Convert temperature to Kelvin.
![\[ n = (PV)/(RT) \]](https://img.qammunity.org/2024/formulas/chemistry/college/c5r86em4utp4bl7j1182kn28nnim0yirm2.png)
![\[ n = \frac{(745 \, \text{mmHg})(1.75 \, \text{L})}{(0.0821 \, \text{L}\cdot\text{atm/mol}\cdot\text{K})(298.15 \, \text{K})} \]](https://img.qammunity.org/2024/formulas/chemistry/college/x4qjwvxxl4tgzydwbgjkm0vzlw8eig8b3l.png)
Therefore, the moles (n) of helium gas is 0.070 mol.
4. **Temperature of Methane:** Use the ideal gas law to find temperature (\(T\)) when given pressure (\(P\)), volume (\(V\)), and mass (\(m\)). Convert pressure to atm.
![\[ T = (PV)/(nR) \]](https://img.qammunity.org/2024/formulas/chemistry/college/o2e5rrdwnl2uyb9wpouhh6uadxm0h4l2oq.png)
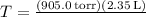
![{(37.2 \, \text{g/mol})(0.0821 \, \text{L}\cdot\text{atm/mol}\cdot\text{K})} \]](https://img.qammunity.org/2024/formulas/chemistry/college/7hg7w2i63ap7hx8nfbukpsje76n1thrctz.png)
Therefore, the temperature T of methane is 694.94 K.
5. **Mass of Ammonia:** Use the ideal gas law to find mass (\(m\)) when given volume (\(V\)), temperature (\(T\)), and pressure (\(P\)). Convert temperature to Kelvin.
![\[ m = (PV)/(RT) \]](https://img.qammunity.org/2024/formulas/chemistry/college/llpxmtldj1tftregz0p1elryixnntdcbh4.png)
![\[ m = \frac{(0.922 \, \text{atm})(0.115 \, \text{L})}{(0.0821 \, \text{L}\cdot\text{atm/mol}\cdot\text{K})(318.15 \, \text{K})} \]](https://img.qammunity.org/2024/formulas/chemistry/college/rwtuj28sb80riqbuell1frfju9b3ad9op7.png)
Therefore, the mass of ammonia is 0.069723g
6. **Density of Ethane Gas:** Calculate density (\(D\)) using the formula \
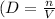 with moles (\(n\)) given, and convert pressure to atm.
with moles (\(n\)) given, and convert pressure to atm.
![\[ D = (n)/(V) \]](https://img.qammunity.org/2024/formulas/chemistry/college/eryzb48vp96ozy6v39geqpw4d27m4deqvg.png)
![\[ D = \frac{2.3 \, \text{mol}}{\frac{194.3 \, \text{kPa}}{101.325 \, \text{kPa/atm}}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/wi7qu05opucq6buij7qvwwjgzuhihhxzh9.png)
Therefore, Density of 2.3 mol of ethane gas is approximately 1.199 mol/L.
7. **Temperature for Gas:** Use the ideal gas law to find temperature (\(T\)) when given volume (\(V\)), pressure (\(P\)), and moles (\(n\)). Convert pressure to atm.
![\[ T = (PV)/(nR) \]](https://img.qammunity.org/2024/formulas/chemistry/college/o2e5rrdwnl2uyb9wpouhh6uadxm0h4l2oq.png)
![\[ T = \frac{(0.750 \, \text{atm})(0.859 \, \text{L})}{(0.0445 \, \text{mol})(0.0821 \, \text{L}\cdot\text{atm/mol}\cdot\text{K})} \]](https://img.qammunity.org/2024/formulas/chemistry/college/muaqagdnysg5zdvjpilxebi9mlqhh9nnv7.png)
Therefore, the temperature (T) is approximately 176.45 K
The probable question may be:
1. 4.3 moles of a gas are at a temperature of 28°C with a pressure of 165.2 kPa. What volume does the gas occupy?
2.163 g CO2 has a volume of 25.12 L at a pressure of 968 mmHg.
What is the temperature of the CO2 in "C?
3.How many moles of helium gas will it take to fill a balloon with a volume of 1.75 L when the temperature is 25°C and the atmospheric pressure is 745 mmHg?
4. At what temperature, in celsius, will 37.2 g of methane, CH4, exert a pressure of 905.0 torr with a volume of 2.35 L
5. determine the mass of a sample ammonia, NH3, that has a volume of 115 mL at a pressure of 0.922 atm and a temperature of 45° C
6. what is the density of 2.3 mol of ethane gas, C2H6, at a pressure of 194.3 kPa and a temperature of 25.6° C?
7. a metal canister has a volume of 859 mL. it’s pressure is 0.750 atm when 0.0445 mol of gas are pumped into the canister . what is the temperature for the gas in K?