Answer:
In a quantum model of the atom, the shape of an electron orbital represents the probability distribution of finding an electron in a particular region of space around the nucleus of an atom. In other words, an electron orbital is a three-dimensional space around the nucleus where there is a high probability of finding an electron with a given energy level.
Different orbitals can have different shapes, such as spherical, hourglass-shaped, or more complex shapes.
Electron Orientations:
s = 1 orbital orientation
p = 3 orientations (
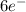 )
)
d = 5 orientations (
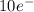 )
)
f = 7 orientations (
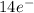 )
)