Final Answer:
The number of hydrogen atoms in 31.30 grams of formaldehyde (CH₂O) is approximately
 atoms.
atoms.
Step-by-step explanation:
Formaldehyde (CH₂O) consists of two hydrogen (H) atoms. To determine the number of moles of CH₂O in 31.30 grams, we use the molar mass of CH₂O, which is calculated by adding the atomic masses of its constituent elements. The molar mass of carbon (C) is
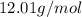 , hydrogen (H) is 1.01 g/mol, and oxygen (O) is 16.00 g/mol. Therefore, the molar mass of CH₂O is \(12.01 + (2 \times 1.01) + 16.00 = 30.03\) g/mol.
, hydrogen (H) is 1.01 g/mol, and oxygen (O) is 16.00 g/mol. Therefore, the molar mass of CH₂O is \(12.01 + (2 \times 1.01) + 16.00 = 30.03\) g/mol.
Next, we calculate the number of moles of CH₂O using the formula:
![\[ \text{Moles} = \frac{\text{Mass}}{\text{Molar Mass}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/qra5gxeovwitk9sf2s6227kgc5fdkvrg70.png)
Substituting the given mass (31.30 g) and molar mass (30.03 g/mol) into the formula, we find that there are approximately \(1.04\) moles of CH₂O.
Since each mole of CH₂O contains two moles of hydrogen atoms, we multiply the number of moles by 2 to find the total number of moles of hydrogen atoms. Therefore:
![\[ \text{Hydrogen Atoms} = 2 * \text{Moles of CH₂O} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/kdipqjgwtesxoiab2pheyyqnh02ittx82b.png)
Substituting the value we obtained earlier, we find that there are approximately \(2.08\) moles of hydrogen atoms. Finally, using Avogadro's number (\(6.022 \times 10^{23}\) atoms/mol), we convert moles to atoms:
![\[ \text{Hydrogen Atoms} \approx 2.08 * 6.022 * 10^(23) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/qo6ew5sxy14apqchjyixnfs20rt4ep1aao.png)
This yields the final answer of approximately
 hydrogen atoms in 31.30 grams of formaldehyde (CH₂O).
hydrogen atoms in 31.30 grams of formaldehyde (CH₂O).