Answer:
10 J
Step-by-step explanation:
Using the idea of the first law of thermodynamics to answer this question.
The first law of thermodynamics simply restates energy conservation: Energy is not created nor is it destroyed it is simply transformed into other forms of energy. It can be stated in the following formula,

In a isothermal process the change in internal energy is equal to zero. So plug in what we know.
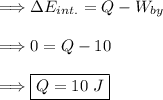
Thus, 10 J of heat must be added to the gas.