Answer:
22.9atm
Step-by-step explanation:
Partial pressures should add to the total pressure. Knowing that we can use the ideal gas law PV=nRT where
P = pressure
V = volume
n = moles of gas
R = gas constant (0.08206
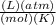 )
)
T = temp in Kelvin (Celcius + 273)
rearranging this formula for pressure we get
P = (nRT)/V
P = ((5.25+4.10)x0.08206x298)/(10.00)
P = 22.9atm