Answer:
3.7996 g
Step-by-step explanation:
From the number of molecules we can find the number of moles of Fluorine gas (F2) and multiply by Fluorine Gas' molecular weight. Fluorine gas is F2,
F = 18.998g/mol.
F2 (g) = 18.998*2 =37.996g F2(g)/mol
1 mol = 6.02 x 10^23 molecules
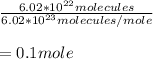
0.1 mol x 37.996g F2 (g) / mol
3.7996 g F2