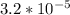Answer:
Ksp =
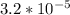
Step-by-step explanation:
If 0.020 M of Ca(OH)2 dissociates, then we can follow the Ksp formula.
Ksp =
![[A]^(a) [B]^(b)](https://img.qammunity.org/2024/formulas/chemistry/high-school/6wyvkeq0lroqpxvlk27z3i7l0pnl8d0gv9.png) Eq.1
Eq.1
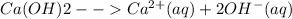 Eq.2
Eq.2
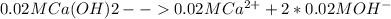
Here, Ca is our A and since it has a coefficient of 1, a = 1
OH is our B. The concentration is doubled because there are 2 moles of OH per mole of Ca(OH)2. Due to this it also has a coefficient of two (Eq.2), making b = 2.
Ksp =
![[0.02][0.02*2]^(2)](https://img.qammunity.org/2024/formulas/chemistry/high-school/t9m7gl1ykiyfwkgyrjh76wvaquauuwd1gx.png)
Ksp = 0.000032
Ksp =