The difference in pressure between ideal and non-ideal conditions for CO₂ is approximately 0.22 atm.
The van der Waals equation for a real gas is given by:
![\[ \left(P + (a)/(V^2)\right)(V - b) = RT \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/pxq7dcklb7je9ywzs697sv2hz9i1fo129a.png)
For the ideal gas equation, PV = nRT, where n is the number of moles.
First, rearrange the van der Waals equation to solve for P:
![\[ P = (RT)/(V - b) - (a)/(V^2) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/xb74w7c9s62y5286yydyni204ojhax7uja.png)
Now, calculate the pressure
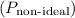 using the van der Waals equation:
using the van der Waals equation:
![\[ P_{\text{non-ideal}} = (RT)/(V - b) - (a)/(V^2) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/8m9cmp5mqnsawdcebjll7s22csnfyye0pc.png)
![\[ P_{\text{non-ideal}} = \frac{(2.0 \, \text{mol})(0.0821 \, \text{L} \, \text{atm} \, \text{mol}^(-1) \, \text{K}^(-1))(200.0 \, \text{K})}{7.30 \, \text{L} - 0.0427 \, \text{L}} - \frac{3.59 \, \text{L}^2 \, \text{atm} \, \text{mol}^(-2)}{(7.30 \, \text{L})^2} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/9v72yok6bvxybel9d16cujv162npl03vhb.png)
![\[ P_{\text{non-ideal}} \approx 4.77 \, \text{atm} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/txyzn44wq5t734rp5t0z4z16feyp9hlx3t.png)
Now, calculate the pressure under ideal conditions
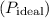 using the ideal gas equation:
using the ideal gas equation:
![\[ P_{\text{ideal}} = (nRT)/(V) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/tapx5cecv9vf3j5qipfrm1z6rkckjxexi3.png)
![\[ P_{\text{ideal}} = \frac{(2.0 \, \text{mol})(0.0821 \, \text{L} \, \text{atm} \, \text{mol}^(-1) \, \text{K}^(-1))(200.0 \, \text{K})}{7.30 \, \text{L}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/yyn173jv29ok3hjc3i2nqf88j3wgefkdfi.png)
![\[ P_{\text{ideal}} \approx 4.99 \, \text{atm} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/wex62blufzqzxga9ijwom9oo4p6i73oe8r.png)
Finally, find the difference in pressure
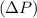 between ideal and non-ideal conditions:
between ideal and non-ideal conditions:
![\[ \Delta P = P_{\text{ideal}} - P_{\text{non-ideal}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/7t3yvjau8rdu7lnkx5y9wnsiv2q9wdiriu.png)
![\[ \Delta P \approx 4.99 \, \text{atm} - 4.77 \, \text{atm} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/qrhdbeyr6762b6bfv347hvh3teuevzlpi4.png)
![\[ \Delta P \approx 0.22 \, \text{atm} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/vivj21ltnek7w3g313628sxkj9b2ii235v.png)
So, the difference in pressure between ideal and non-ideal conditions for CO₂ is approximately 0.22 atm.