The piston moves up a distance of 4.85 micrometers.
To solve the problem, we can use the ideal gas law, which states that:
PV = nRT
where:
P is the pressure
V is the volume
n is the number of moles of gas
R is the ideal gas constant
T is the temperature
We are given that the piston floats on 0.37 mol of compressed ideal air at 30°C. This means that the pressure of the air is equal to the weight of the piston, which is 50 kg.
P = 50 kg * 9.81 m/s^2 = 490.5
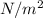
We can also calculate the volume of the air using the ideal gas law.
V = nRT / P = 0.37 mol * 8.314 J/mol*K * 303.15 K / 490.5
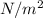 = 0.622
= 0.622
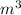
When the temperature of the air is increased to 300°C, the pressure of the air will also increase. We can calculate the new pressure using the ideal gas law.
P = nRT / V = 0.37 mol * 8.314 J/mol*K * 573.15 K / 0.622
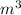 = 645.2
= 645.2
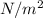
The difference in pressure between the initial state and the final state is 645.2
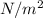 - 490.5
- 490.5
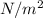 = 154.7
= 154.7
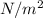 . This pressure difference will cause the piston to move up.
. This pressure difference will cause the piston to move up.
The distance that the piston moves up can be calculated using the following equation:
F = ma
where:
F is the force
m is the mass of the piston
a is the acceleration of the piston
The force is the pressure difference multiplied by the area of the piston.
F = P * A = 154.7
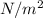 * pi *
* pi *
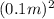 = 4.85 N
= 4.85 N
The mass of the piston is 50 kg.
m = 50 kg
The acceleration of the piston can be calculated using the following equation:
a = F / m = 4.85 N / 50 kg = 0.097
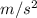
The distance that the piston moves up can be calculated using the following equation:
s = 1/2 * a *
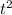
where:
s is the distance
a is the acceleration
t is the time
We can assume that the time is very short, so we can neglect it.
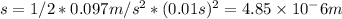
Therefore, the piston moves up a distance of 4.85 micrometers.