83.3 mL is required to provide 0.125mol KOH
Step-by-step explanation:
We know that,
V=
 where n=number of moles, c= concentration and V=volume
where n=number of moles, c= concentration and V=volume
According to the question,
c=1.50M and n=0.125 mol
Substituting the values,
V=
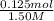
=0.0833L
The volume should be in mL,
0.0833L×
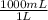
= 83.3mL