Boyle's Law-

(Pressure is inversely proportional to the volume)
Where-
 = Initial volume
= Initial volume
 = Final volume
= Final volume
 = Initial pressure
= Initial pressure
 = Final pressure
= Final pressure
As per question, we are given that -
Now that we have all the required values and we are asked to find out the final volume, so we can put the values and solve for the final volume -

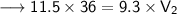
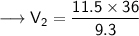
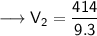
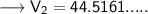

Therefore, the volume of the ammonia will become 44.52 L if its pressure is changed to 9.3 kPa while its temperature remains constant.