Gay-Lussac's Law-
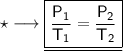

Where-
- P₁ is the initial pressure.
- T₁ is the initial temperature
- P₂ is the final pressure.
- T₂ is the final temperature
As per question, we are given -
- P₁ = 6.8 KPa
- T₁ =539 K
- T₂= 211K
Now that we are given all the required values, so we can put them into the formula and solve for P₂:-
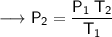
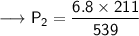
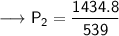
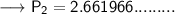
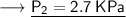
Therefore, If the temperature decreases to 211K, then the new pressure will become 2.7 KPa.