Boyle's Law-

(Pressure is inversely proportional to the volume)
Where-
 = Initial volume
= Initial volume
 = Final volume
= Final volume
 = Initial pressure
= Initial pressure
 = Final pressure
= Final pressure
As per question, we are given that -
 = 750 mL
= 750 mL
 = 2.56 atm
= 2.56 atm
 = 985 mL
= 985 mL
Now that we have all the required values and we are asked to find out the final pressure, so we can put the values and solve for the final pressure of nitrogen -


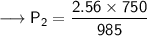
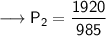
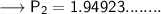

Therefore,the pressure will become 1.95 atm if the volume becomes 985mL.