Answer:
4.117703084×10²² molecule
Step-by-step explanation:
According to the definition of the mole which is a unit of amount of substance which contains exactly 6.02214076×10²³ (Avogadro's Number
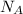 )elementary entities
)elementary entities
The formula of calculating number of moles for a given mass of a specified chemical species:
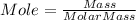
Where Molar mass: the mass in grams of one mole of the compound (unit is g/mol or gram/mole)
The formula of calculating number of particles for a given number of moles:
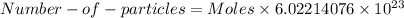
So, to calculate the moles of NaCl, we need to calculate the molar mass first
The molar mass of NaCl = Molar mass of Cl + Molar mass of Na = 35.5 + 23 = 58.5 g/mol
No. of moles in 4 grams of NaCl = 4/58.5 = 0.0684 moles
Then, We calculate the number of molecules in 0.0684 moles of NaCl
Which =
0.0684 × 6.02214076×10²³ = 4.117703084×10²² molecule
Have any questions? Write in the comments