Answer:
The final volume of gas in the balloon is 40.0 L (nearest tenth).
Step-by-step explanation:
To solve this problem we can use the Combined Gas Law.
Combined Gas Law
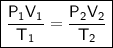
where:
- P₁ is the initial pressure.
- V₁ is the initial volume.
- T₁ is the initial temperature (measured in kelvin).
- P₂ is the final pressure.
- V₂ is the final volume.
- T₂ is the final temperature (measured in kelvin).
Convert the temperatures given in Celsius to kelvin by adding 273.15:
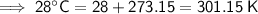
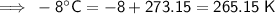
Therefore, the values to substitute into the formula are:
- P₁ = 745 torr
- V₁ = 30.2 L
- T₁ = 301.15 K
- P₂ = 495 torr
- T₂ = 265.15 K
Substitute the values into the formula and solve for V₂:
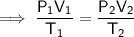
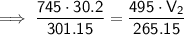
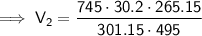
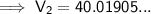
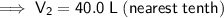
Therefore, the final volume of gas in the balloon is 40.0 L (nearest tenth).