Answer :
Atomic mass of C (Carbon) = 12
Atomic mass of O (Oxygen) = 16.
Atomic mass of
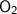 = 16 × 2 = 32
= 16 × 2 = 32
Atomic mass of
 = Atomic mass of C +Atomic mass of
= Atomic mass of C +Atomic mass of
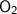
Atomic mass of
 = 12 + 32
= 12 + 32
Atomic mass of
 = 44 u
= 44 u
Atomic mass of first 20 elements :
1. (H) Hydrogen - 1.00797
2. (He) Helium - 4.00260
3. (Li)Lithium- 6.941
4. (Be) Beryllium- 9.01218
5. (B) Boron- 10.81
6. (C) Carbon- 12.011
7 . (N) Nitrogen- 14.0067
8. (O) Oxygen- 15.9994
9. (F) Fluorine- 18.998403
10. (Ne) Neon- 20.179
11. (Na) Sodium- 22.98977
12.(Mg)Magnesium- 24.305
13. (Al) Aluminum- 26.98154
14. (Si) Silicon -28.0855
15. (P) Phosphorus- 30.97376
16. (S) Sulfur- 32.06
17. (Cl) Chlorine- 35.453
18.(Ar)Argon- 39.948
19. (K) Potassium- 39.0983
20. (Ca) Calcium- 40.0