The density of Cube A, which sinks in water, is approximately
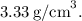 This is calculated by dividing its mass (90 g) by its volume (27 cm³).
This is calculated by dividing its mass (90 g) by its volume (27 cm³).
In the scenario presented, Cube A, with dimensions 3 cm x 3 cm x 3 cm and a mass of 90 grams, is found to sink in water. To determine the density of Cube A, one can employ the formula:
![\[ \text{Density} = \frac{\text{Mass}}{\text{Volume}} \].](https://img.qammunity.org/2024/formulas/physics/college/z6lc0ay3tzccc2wd1j8esw1n0accsl73mh.png) The volume of Cube A, calculated as
The volume of Cube A, calculated as

The probable question maybe:
If Cube A, with dimensions 3 cm x 3 cm x 3 cm and a mass of 90 grams, sinks in water, what is the density, in grams per cubic centimeter, of Cube A?