a. Set up a concentration cell with Mg²⁺ electrodes to measure activity in a 0.100 M ATP solution. b. Measure thermodynamic equilibrium constant for Mg²⁺ binding to ATP using an electrochemical cell with equilibrium conditions and Nernst equation.
a. To measure the activity of Mg²⁺ in a 0.100 M ATP solution, you can set up an electrochemical cell known as a concentration cell. A concentration cell utilizes the difference in concentration of ions between two half-cells to generate a voltage that can be measured. The cell consists of two half-cells with the same electrodes but different concentrations of the ions of interest.
Here's a suggested setup:
1. Half-Cell 1 (Higher [Mg²⁺]):
- Electrode: Mg²⁺ | Mg
- Solution: 0.100 M ATP + x M Mg²⁺
2. Half-Cell 2 (Lower [Mg²⁺]):
- Electrode: Mg²⁺ | Mg
- Solution: 0.100 M ATP
The Mg²⁺ concentration in Half-Cell 1 is higher than in Half-Cell 2 due to the addition of x M Mg²⁺. The potential difference between the two half-cells will be related to the concentration difference of Mg²⁺ ions. By measuring the cell potential, you can determine the activity of Mg²⁺ in the 0.100 M ATP solution.
b. To measure the thermodynamic equilibrium constant (K) for the binding of Mg²⁺ by ATP, you can set up an electrochemical cell that operates under equilibrium conditions. This would involve a cell where the concentrations of reactants and products do not change over time.
Here's a suggested setup:
1. Half-Cell 1 (Higher [Mg-ATP]):
- Electrode: Mg²⁺ | Mg-ATP
- Solution: 0.100 M ATP + x M Mg²⁺
2. Half-Cell 2 (Lower [Mg-ATP]):
- Electrode: Mg²⁺ | Mg-ATP
- Solution: 0.100 M ATP
In this case, you have Mg²⁺ reacting with ATP to form Mg-ATP. At equilibrium, the rate of the forward reaction (Mg²⁺ binding to ATP) is equal to the rate of the reverse reaction (Mg-ATP dissociating). The cell potential at equilibrium reflects the thermodynamic equilibrium constant (K) for the binding reaction.
By measuring the cell potential and applying the Nernst equation, you can calculate the equilibrium constant (K) for the binding of Mg²⁺ by ATP. The Nernst equation is given by:
![\[ E_{\text{cell}} = E^\circ_{\text{cell}} - (RT)/(nF) \ln Q \]](https://img.qammunity.org/2024/formulas/chemistry/college/1yhwnjkl1b8kojf97bqiypvlubocyffjz2.png)
Where:
-
 is the cell potential at equilibrium.
is the cell potential at equilibrium.
-
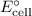 is the standard cell potential.
is the standard cell potential.
-
 is the gas constant.
is the gas constant.
-
 is the temperature in Kelvin.
is the temperature in Kelvin.
-
 is the number of electrons transferred.
is the number of electrons transferred.
-
 is Faraday's constant.
is Faraday's constant.
-
 is the reaction quotient.
is the reaction quotient.