Final Answer:
The pH is 9.00.
The correct option is c.
Step-by-step explanation:
The pH of a solution containing a weak base, such as the acetate ion (CH₃COO⁻), can be determined using the formula:
![\[ \text{pOH} = -\log_(10)(K_b) - \log_(10)(\text{concentration of base}) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/vrsatemn5m4y151xlpc25xoqae958pcnbt.png)
Since
 is the base dissociation constant and the acetate ion is the conjugate base of acetic acid (CH₃COOH), we can use the
is the base dissociation constant and the acetate ion is the conjugate base of acetic acid (CH₃COOH), we can use the
 of acetic acid in the formula:
of acetic acid in the formula:
![\[ \text{pOH} = -\log_(10)(K_a) - \log_(10)(\text{concentration of acetate ion}) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/gq8hgpy6q2s9qc57q9fvyq2p56hd4ykfoy.png)
Given
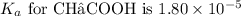 , and the concentration of acetate ion is 0.100 moles in 1 liter (0.100 M), we substitute these values into the formula:
, and the concentration of acetate ion is 0.100 moles in 1 liter (0.100 M), we substitute these values into the formula:
![\[ \text{pOH} = -\log_(10)(1.80 * 10^(-5)) - \log_(10)(0.100) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/6rqvuwduue46o4ywk3od0rttoy8agtrdor.png)
Now, calculate the pOH and then find the pH using the relationship
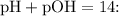
![\[ \text{pOH} \approx -\log_(10)(1.80 * 10^(-5)) - \log_(10)(0.100) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/hea27kkvw0iy4r8qz1bnauyyips555igze.png)
![\[ \text{pH} \approx 14 - \text{pOH} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/nb30ilw2vtp1pyu6jj9m1tvml6fziroswi.png)
After calculation, the pH is approximately 9.00, which corresponds to option c. Therefore, the final answer is 9.00. This indicates that the solution is slightly basic.
The correct option is c.