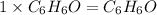Answer: The molecular formula will be
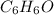
Step-by-step explanation:
Molecular formula is the chemical formula which depicts the actual number of atoms of each element present in the compound.
Empirical formula is the simplest chemical formula which depicts the whole number of atoms of each element present in the compound.
The empirical formula is
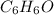
The empirical weight of
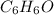 = 6(12)+6(1)+1(16) = 94 g.
= 6(12)+6(1)+1(16) = 94 g.
The molecular weight =
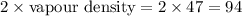
Now we have to calculate the molecular formula.
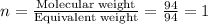
The molecular formula will be=