The number of mole of aluminum nitrate that can be produced from the reaction is 0.0077 mole
How to calculate the number of mole of aluminum nitrate produced?
The number of mole of aluminum nitrate,
 produced when 3 grams of barium nitrate,
produced when 3 grams of barium nitrate,
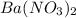 reacts with aluminum hydroxide,
reacts with aluminum hydroxide,
 can be calculated as shown below:
can be calculated as shown below:
- Mass of barium nitrate,
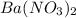 (m) = 3 grams
(m) = 3 grams - Molar mass of barium nitrate,
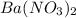 (M) = 261.337 g/mol
(M) = 261.337 g/mol - Mole of barium nitrate,
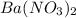 = m / M = 3 / 261.337 = 0.0115 mole
= m / M = 3 / 261.337 = 0.0115 mole - Mole of aluminum nitrate,
 produced =?
produced =?
Balanced equation for the reaction:
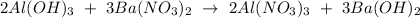
From the equation above,
3 moles of barium nitrate,
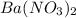 reacted to produce 2 moles of aluminum nitrate,
reacted to produce 2 moles of aluminum nitrate,

Therefore,
0.0115 mole of barium nitrate,
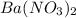 will react to produce =
will react to produce =
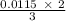 = 0.0077 mole of aluminum nitrate,
= 0.0077 mole of aluminum nitrate,

Thus, the mole of mole of aluminum nitrate,
 produced from the reaction is 0.0077 mole
produced from the reaction is 0.0077 mole