Answer:
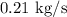
Step-by-step explanation:
P = Pressure =
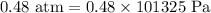
V = Volume =
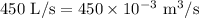
R = Gas constant =
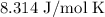
T = Temperature =
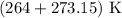
The reaction is
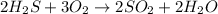
From ideal gas equation we have
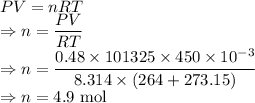
Moles of
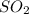 produced is
produced is
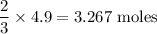
Molar mass of
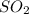 = 64.066 g/mol
= 64.066 g/mol
Production rate is
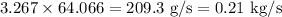
The rate at which sulfur dioxide is being produced
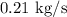 .
.