Answer:
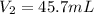
Step-by-step explanation:
Hello there!
In this case, since the equation to deal with dilution process involves the initial and final concentration and volume:
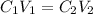
Thus, given the initial and final concentrations and the initial volume, we can write:
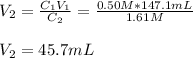
Best regards.