Final Answer:
The molar solubility
 of the salt
of the salt
 in a solution containing
in a solution containing
 is determined by the expression
is determined by the expression
 is the solubility product constant. Solving for
is the solubility product constant. Solving for
 yields the molar solubility.
yields the molar solubility.
Step-by-step explanation:
To find the molar solubility
 of the salt
of the salt
 , we start with the equilibrium expression for the dissolution of the salt:
, we start with the equilibrium expression for the dissolution of the salt:
![\[ MX \rightleftharpoons M^(+)_x + xX^(-) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/y9k9i696mkckxqs0tlq3vxpzy7xpuoshem.png)
The equilibrium constant
 for this reaction is expressed as
for this reaction is expressed as
![\(K_{\text{sp}} = [M^(+)]^x [X^-]^x\)](https://img.qammunity.org/2024/formulas/chemistry/high-school/b6vlcfzroexrs6pmvix9q0vqss5xh1nh9e.png) . Since the stoichiometry is 1:1 for
. Since the stoichiometry is 1:1 for
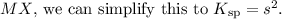
Now, the solution already contains
 which contributes to the concentration of
which contributes to the concentration of
 ions. Thus, the equilibrium concentration of
ions. Thus, the equilibrium concentration of
 . Substituting this into the
. Substituting this into the
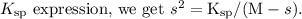
Solving this quadratic equation for
 we obtain the molar solubility. It's important to note that this approach considers the common ion effect from the initial concentration of
we obtain the molar solubility. It's important to note that this approach considers the common ion effect from the initial concentration of
 . Understanding these principles is fundamental in predicting the behavior of salts in solution.
. Understanding these principles is fundamental in predicting the behavior of salts in solution.
Lastly, calculating
 provides insights into the saturation point of the solution, helping to assess potential precipitation or the formation of complexes.
provides insights into the saturation point of the solution, helping to assess potential precipitation or the formation of complexes.
The complete question is:
"What is the molar solubility (in mol L⁻¹) of a salt with the general molecular formula
 is a cation and
is a cation and
 is an anion) in a solution that initially contains
is an anion) in a solution that initially contains
 ? Given the solubility product constant
? Given the solubility product constant
 determine the equilibrium concentration of
determine the equilibrium concentration of
 in the solution."
in the solution."