The ion that does not have [Xe] as its electronic configuration is
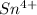 . Option D
. Option D
What is the configuration?
Sn4+ is the only ion among the ones provided whose electronic configuration does not include [Xe]. Tin (Sn) has the electronic configuration [Kr] 4d¹⁰ 5s² 5p²; it achieves this configuration when it loses four electrons to form the
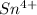 ion.
ion.
In contrast, after gaining or losing electrons, the ions
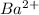 ,
,
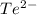 ,
,
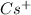 , and
, and
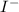 all reach configurations that include [Xe]. In their individual ion configurations, the noble gas xenon's electronic structure is retained by the (barium), (tellurium), (celesium), and (iodine) ions.
all reach configurations that include [Xe]. In their individual ion configurations, the noble gas xenon's electronic structure is retained by the (barium), (tellurium), (celesium), and (iodine) ions.