Answer:
68.95moles
Step-by-step explanation:
Given parameters:
Number of particles of lithium = 4.151 x 10²⁵ particles
Unknown:
Number of moles = ?
Solution:
From the mole concepts, we know that;
6.02 x 10²³ particles are contained in 1 mole of a substance
4.151 x 10²⁵ particles contains
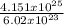 = 68.95moles
= 68.95moles