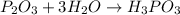Your answer is
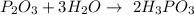
but if you are looking for some explanation check description below :)
Diphosphorus trioxide formula is:
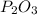
Di stands from 2 atoms of something, in this case we have 2 atoms of Phosphorus.
Trioxide means that we have 3 atoms of oxygen in this substance that's why we have:
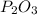
And water is just

Unbalanced formula is:
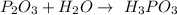
Now we need to balance this equation
On left hand side we have 2 atoms of Phosphorus, on right hand side we have just one atom of Phosphorus, that's why we need to put 2 before
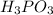
Now we need to count all atoms of oxygen and hydrogen and make it equal on both side.
So we need to put 3 on left hand side before water to make this equation balanced.
That's why correct answer is: