Step 1 is correct.
Step 2.
There are 2 Iron (Fe) atoms in the formula so we will multiply 2 with the relative mass of Fe .
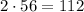
Relative mass of Iron atoms in the formula is 112 amu (atomic mass unit)
Step 3.
To find the percentage,
we will divide mass of iron to total mass and equal that to x divided to 100.
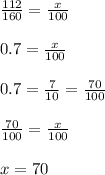
Total mass of iron is 70% of the total mass of the formula.