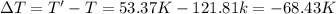Answer:The change in temperature of the gas mixture is -68.43 K.
Initial temperature of the gas 121.81 K.
Step-by-step explanation:
Initial temperature of the container with 2.50 L of volume of helium gas alone:T
Initial pressure ,P = 1.83 atm
number of moles of helium ,n= 0.458 mol
V = 2.50 L
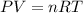 ( Ideal gas equation)
( Ideal gas equation)
R = universal gas constant = 0.0820 atm L/mol K
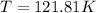
Initial temperature of the gas 121.81 K.
Final temperature of the container with 2.50 L of volume with helium and argon gas:T'
Final pressure ,P' = 2.05 atm
Number of moles of helium ,n'= 0.713 mol + 0.458 mol = 1.171 mol[/tex]
V = 2.50 L
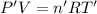
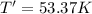
The change in temperature of the gas mixture