Answer :
 reactant is reduced and
reactant is reduced and
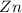 reactant is oxidized.
reactant is oxidized.
Explanation :
Redox reaction : It is a type of reaction in which an oxidation and reduction reaction takes palace simultaneously.
Oxidation : The loss of electrons or increase in the oxidation number is an oxidation.
Reduction : The gain of electrons or decrease in the oxidation number is a reduction.
The given balanced redox reaction is,
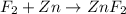
The oxidation number of
 = 0
= 0
The oxidation number of
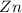 = 0
= 0
The oxidation number of Zn in
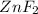
x + 2(-1) = 0
x = +2
The oxidation number of
 in
in
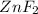 = -1
= -1
That means
 is reduced due to decrease in the oxidation number from 0 to (-1) and 'Zn' is oxidized due to increase in the oxidation number from 0 to (+2).
is reduced due to decrease in the oxidation number from 0 to (-1) and 'Zn' is oxidized due to increase in the oxidation number from 0 to (+2).
Therefore,
 reactant is reduced and
reactant is reduced and
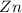 reactant is oxidized.
reactant is oxidized.