Answer:
2.86L
Step-by-step explanation:
Given parameters:
V1 = 2.1L
T1 = 200°C = 200 + 273 = 473K
T 2 = 370°C = 370 + 273 = 643K
Unknown:
V2 = ?
Solution:
Since pressure is constant, we apply the Charles's law;
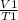 =
=
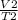
V is volume
T is the temperature
1 is the initial state
2 is the new state
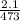 =
=
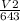
V2 = 2.86L