Answer: Option (a) is the correct answer.
Step-by-step explanation:
As the given atom is
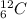 so, it means that it contains 6 protons. Also, when an atom is neutral then its number of protons equal to the number of electrons.
so, it means that it contains 6 protons. Also, when an atom is neutral then its number of protons equal to the number of electrons.
Atomic mass means the sum of total number of protons and neutrons present in an atom. As
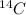 is an isotope of
is an isotope of
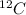 . And, all the isotopes of carbon or any other element contains the same number of protons but different number of neutrons.
. And, all the isotopes of carbon or any other element contains the same number of protons but different number of neutrons.
Therefore, there will be 6 protons present in
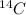 .
.
Thus, we can conclude that carbon-14 atom have 6 protons.