First, find the concentration of the NaCN solution in terms of molarity. The molar mass of NaCN is 49.0072 g/mol
Molarity = mol solute ÷ L solution
Molarity = (353 mg * (1 g / 1000 mg) * (1 / 49.0072 g/mol)) ÷ (500 mL * (1 L / 1000 mL))
Molarity = 0.0144 M NaCN
NaCN is a salt from a weak acid HCN and a strong base NaOH. The reaction is as follows:
HCN + NaOH --> NaCN + H2O
Upon hydrolysis, it becomes:
 +
+
 --> HCN +
--> HCN +

Initial: Amount of
 is 0.0144 M,
is 0.0144 M,
 is in excess, and the products are 0.
is in excess, and the products are 0.
Change: This is unknown. Let this be x. For the
 , it would be –x, while it would be +x for the products HCN and
, it would be –x, while it would be +x for the products HCN and
 .
.
Excess: This would be Initial + Change. Thus for
 , it would be 0.0144 – x. For both products, it would be x for each.
, it would be 0.0144 – x. For both products, it would be x for each.
The hydrolysis constant (
 ) would be the amount of products over the reactants. Thus,
) would be the amount of products over the reactants. Thus,
 = (HCN)*(
= (HCN)*(
 ) / (
) / (
 ) = x2 / (0.0144 –x) --> equation 1
) = x2 / (0.0144 –x) --> equation 1
 is also equal to the equilibrium constant of water (
is also equal to the equilibrium constant of water (
 = 1 x 10^-14) divided by
= 1 x 10^-14) divided by
 .
.
 = 1 x
= 1 x
 ÷ 2.1 x
÷ 2.1 x
 --> equation 2
--> equation 2
Substituting equation 2 to equation 1:
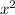 ÷ (0.0144 –x) = 4.762 x
÷ (0.0144 –x) = 4.762 x

Solving for x,
X = 2.59 x

As mentioned before, x denotes the products HCN and
 . Thus the amount of
. Thus the amount of
 is 2.59 x
is 2.59 x

To find pH,
pH = 14-(-log
 )
)
pH = 14 – (-log 2.59 x
 )
)
pH = 10.41
Thus, the answer is 10.41.