Answer:
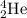
Step-by-step explanation:
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons
In an atom, when neutrons or protons are lost or gains, it directly affects the mass number of an atom.
Atomic number is defined as the number of protons or number of electrons that are present in an atom.
Atomic number = Number of electrons = Number of protons
General representation of an element is given as:

where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
1.
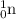 has 0 proton and 1 neutron.
has 0 proton and 1 neutron.
2.
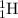 has 1 proton and zero neutrons.
has 1 proton and zero neutrons.
3.
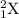 has 1 proton and 1 neutron.
has 1 proton and 1 neutron.
4.
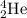 has 2 protons and 2 neutrons.
has 2 protons and 2 neutrons.