Answer:
The correct answer is option C.
Step-by-step explanation:
Half life of the uranium-238=
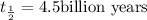
Decay constant =

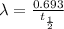
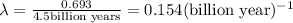
Let the initial amount of U-238 be x
And the present amount of U-238 be x'.
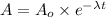
 = Initial amount
= Initial amount
A = Amount left after time t
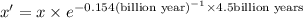
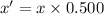
Percentage of left amount:

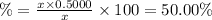
Hence,the correct answer is option C.