Answer:
Copper (Cu)
Atomic Number: 29
Element Category: Transition metal
Electron Configuration:
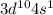
Sulfur (S)
Atomic Number: 16
Element Category: Reactive nonmetal
Electron Configuration:
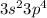
Copper Sulfide (Cu
 S
S
 )
)
Step-by-step explanation:
Copper sulfides will always vary in composition (0.5 ≤ Cu/S ≤ 2) because their bonds are somewhat covalent and have complicated electronic band structures because of their high degree of delocalization.