Answer: The percent sulfur in the compound is 6.308 %.
Step-by-step explanation:
We are given:
Mass of barium sulfate = 1.1756 g
We know that:
Molar mass of barium sulfate = 233.38 g/mol
Molar mass of sulfur atom = 32.07 g/mol
As, all the sulfur in the compound is converted to barium sulfate. So, the mass of sulfur in barium sulfate will be equal to the mass of sulfur present in the compound.
To calculate the mass of sulfur in given mass of barium sulfate, we apply unitary method:
In 233.38 g of barium sulfate, mass of sulfur present is 32.07 g
So, in 1.1756 g of barium sulfate, mass of sulfur present will be =
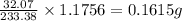
To calculate the percentage composition of sulfur in compound, we use the equation:
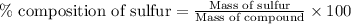
Mass of compound = 2.5600 g
Mass of sulfur = 0.1615 g
Putting values in above equation, we get:
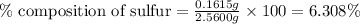
Hence, the percent sulfur in the compound is 6.308 %.