Answer:
C₆H₆
Step-by-step explanation:
Molar mass of the compound is 78g/mol
Unknown:
Molecular formula of the compound = ?
Solution:
The empirical formula of the compound is CH.
Empirical formula is the simplest formula of a compound.
To solve this problem:
Find the molar mass of CH;
Molar mass = 12 + 1 = 13g/mol
Multiplication index =
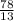 = 6
= 6
So;
(CH)₆ ;
Therefore:
C₆H₆